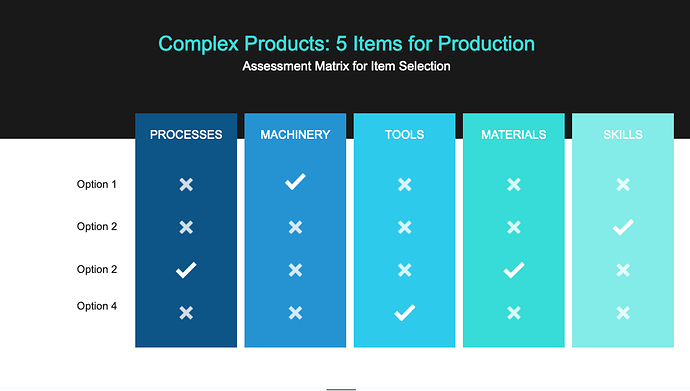
Jumping onto this thread with my own comments, for what it’s worth haha. So far, I agree with the jist of this thread - the distinction between medical and healthcare is not only flexible and avoids overselling highly regulated and specialised equipment, but also aids the central purpose of the exercise to test distributed production. It seems fair that in the assesment matrix we include the goals of the testing of distributed manufacturing, such as a range of materials, machines and distributed infrastructure applied across the product categories.
If it helps expanding the range of candidate products, it seems to me a clinic might also need less medicalised products, like toys for children, building maintenance (spare door knobs, I don’t know) and signage - which might improve the healthcare experience, patient turnover and staff workflows.
Regarding assistive designs, say grab handles or mobility devices, even if they’re less regulated than medical devices there are still detailed ergonomic considerations that apply to disabled, elderly, expectant mothers, children etc. Here’s a reference I’ve used before for inclusive design, which would detail the kind of requirements for a product for disability (https://www.routledge.com/Extra-Ordinary-Ergonomics-How-to-Accommodate-Small-and-Big-Persons-The/Kroemer/p/book/9780367392321) (Happy to scan in my copy and share pdfs haha). Not exactly sure how exactly this point applies here, except as a selection criteria for appropriately considered designs, ie. if you have a product intended for use by pregnant people, has the appropriate anthropometric data been used in the tolerancing?
But, if you need to delimit the range of products right down to a handful of ideas, rather than widen the selection pool and making the job harder, then a quick phone call to a clinic bluntly asking ‘what do you need6’ could work wonders.
Relating to my recent research on materials (shameless plug: Scoping Document for Materials and Components - Draft Available for Comments) , it seems to me that setting out as a selection critereon material availability from the outset would be hard - you’d end up in a quagmire of local suppliers, varying vernacular etc. etc. Instead, this is a golden opportunity to use as a product selection critereon a highly detailed material specification, then ask the makerspaces to report on the materials actually used and any substitutions made, as a kind of pseudo inspection certificate. This way, an outcome of the trials could be an intuative sense of material availability, after the fact.
Hope any of this helps  Really interesting discussion here, hope the trials go well. Best of luck to the makerspaces and clinics
Really interesting discussion here, hope the trials go well. Best of luck to the makerspaces and clinics